www.drvaibhavshah.net
www.drvaibhavshah.net is website of Dr. Vaibhav Shah who is Facial Cosmetic & Hair Transplant Surgery.
 |
| Dr Vaibhav Shah |
www.drvaibhavshah.net is website of Dr. Vaibhav Shah who is Facial Cosmetic & Hair Transplant Surgery.
www.drvaibhavshah.net
is more like knowledge sharing website , it helps patients to understand
Different Cosmetic Surgery & procedures in detailed. Website focuses on
different
Indication,
How it can be performed?
Right Candidate for Procedure,
Patient Selction,
What to expect from Surgery or Procedure,
Possible Results
. . . & so on . . . ..
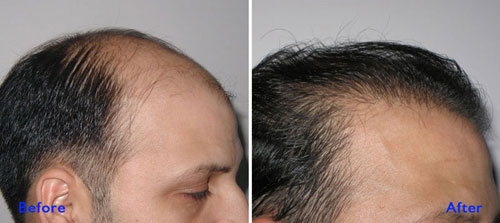
There is separate Before – After Gallery of Patients
which gives general Idea what one should expect after Procedure or Surgery.
In general its complete , Informative, Knowledge
sharing tool for patients to understand about
Hair Transplant Surgery
Facial Cosmetic Surgery.